Chemistry Mug, 1 Mole per Liter, 1 Mole/liter, Science Teacher Gifts, Mol per Litre Coffee Cup, Funny Science Mug, Chemistry Pun - Etsy UK
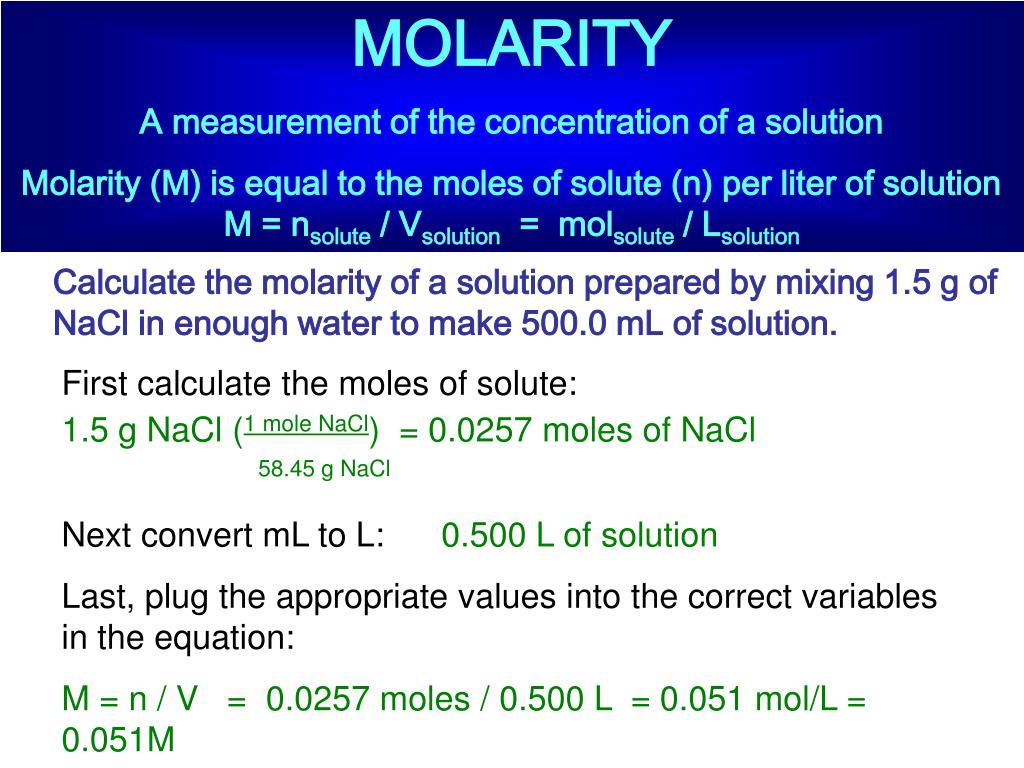
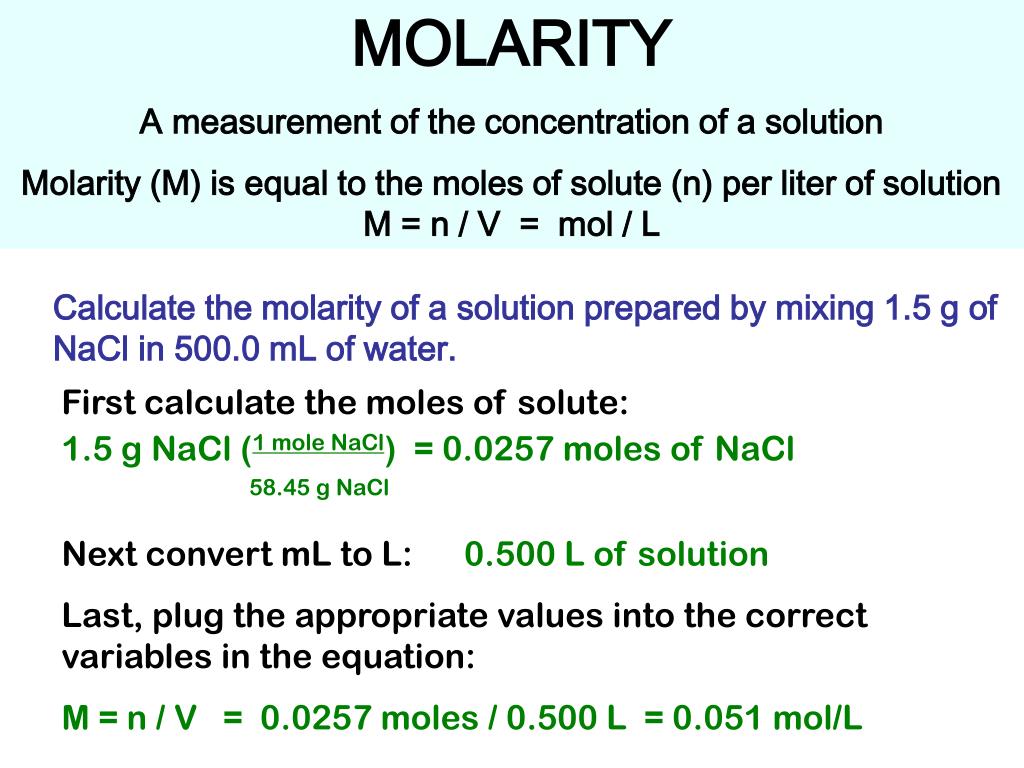
PPT - MOLARITY A measurement of the concentration of a solution PowerPoint Presentation - ID:1459950
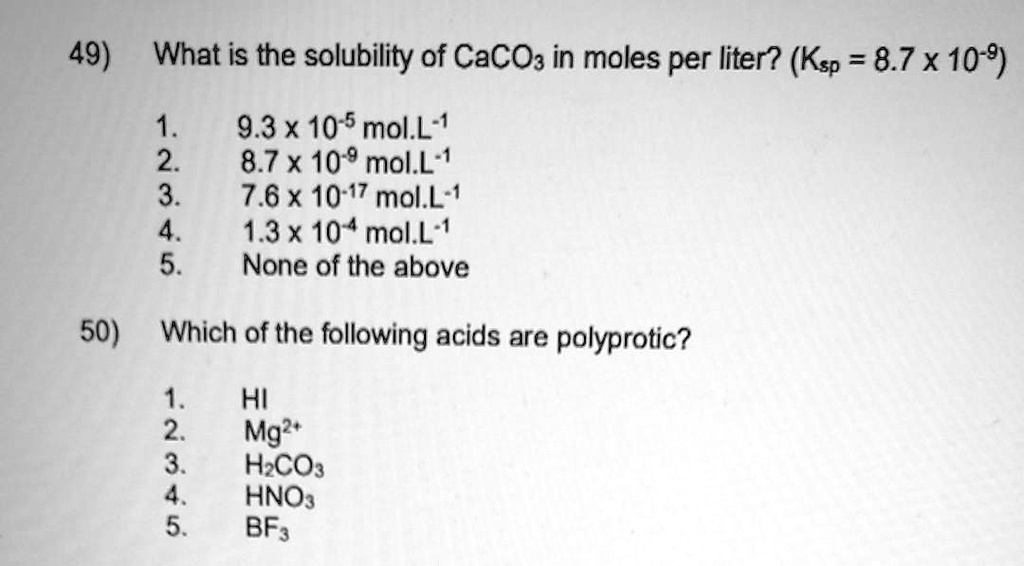
SOLVED: 49) What is the solubility of CaCO3 in moles per liter? (Ksp = 8.7 x 10^-9) 1 9.3 x 10^5 mol.L^-1 2 8.7 x 10^-9 mol.L^-1 3 7.6 x 10^-17 mol.L^- 1

Sticker for Sale mit "Chemie 1 Mol pro Liter für den Tag des Maulwurfs oder des Avogadro" von SAFREN | Redbubble
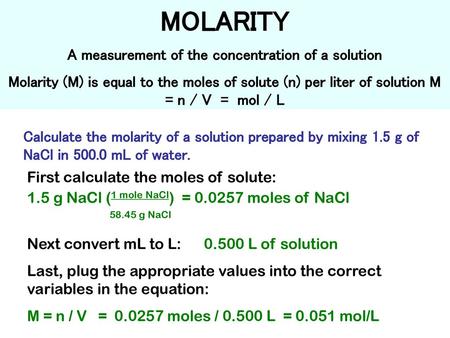
MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of solution M = mol / L Calculate. - ppt download
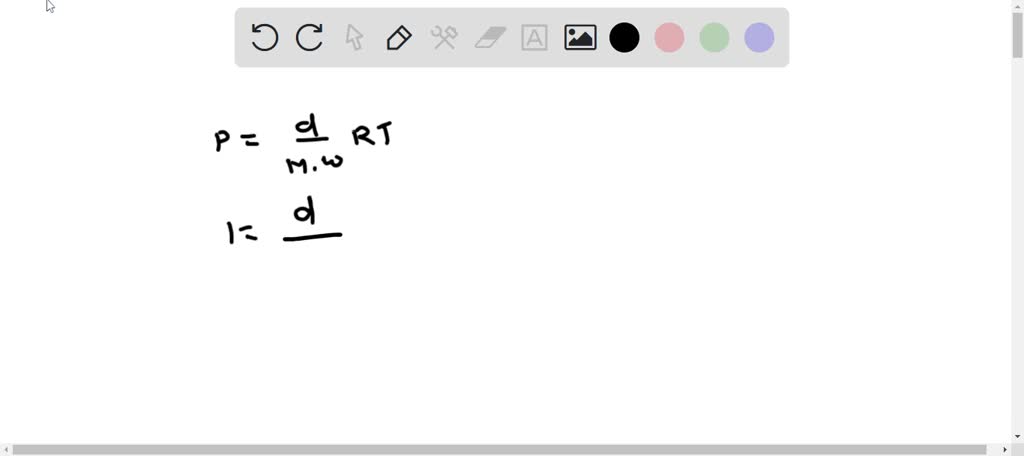
SOLVED: What is the approximate density of 1 mole of fluorine gas, F2, (molar mass = 70.1 g/mol) in units of grams per liter at STP, given that 1 mol = 22.414

Kimia 1 Mol Per Liter untuk Mol atau Mug Teh Sederhana Perlengkapan Minum Hadiah Foto Pegangan Bulat Cangkir Dicetak Gambar Desain Kopi - AliExpress

Chemistry Mug, 1 Mole per Liter, 1 Mole/liter, Science Teacher Gifts, Mol per Litre Coffee Cup, Funny Science Mug, Chemistry Pun - Etsy
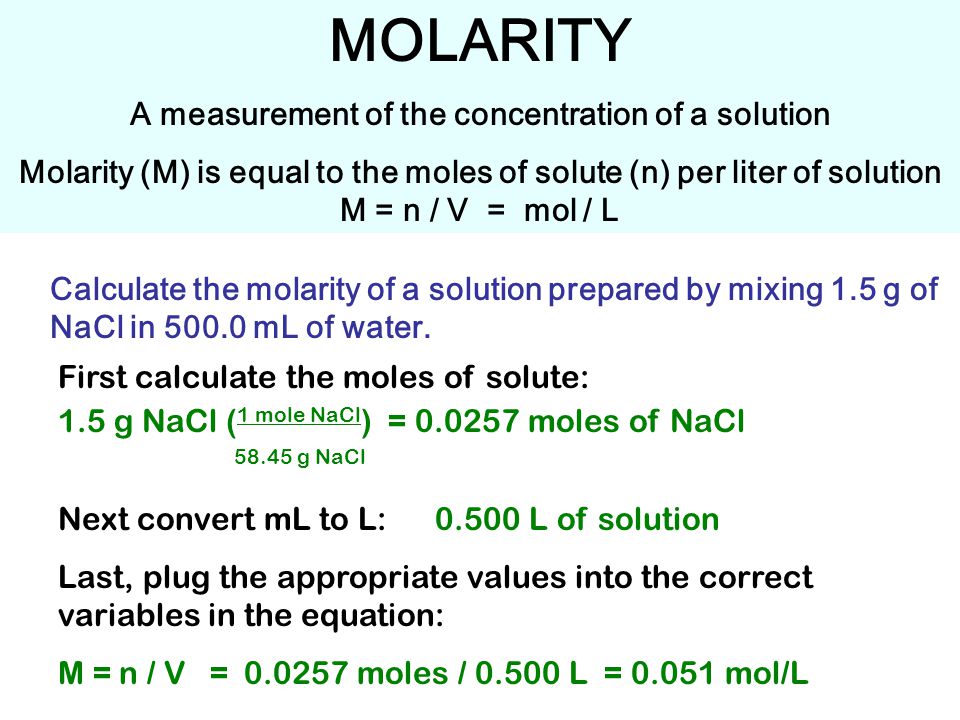
MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of solution M = n / V = mol / L Calculate. - ppt download
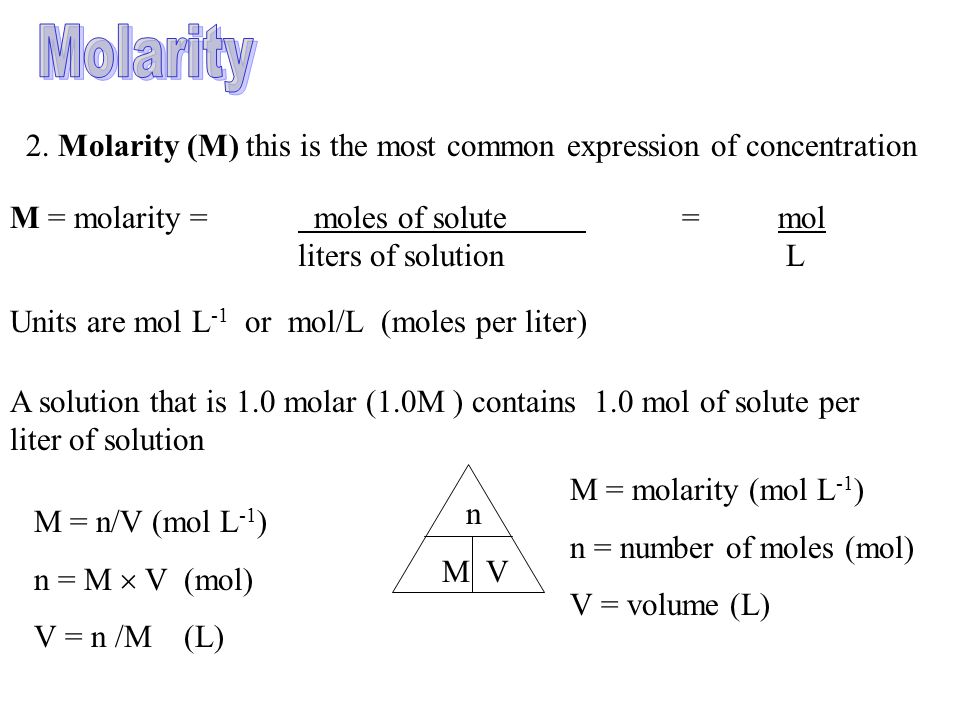
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download