Enthalpy of combustion of alkanes graph vales versus carbon number molecular mass Complete & incomplete combustion of alkanes environmental pollution problems advanced A level organic chemistry revision notes
The combustion enthalpies of carbon, hydrogen, and methane are 395.5 kJ mol^ 1, 284.8 kJ mol^ 1 and 890.4 kJ mol^ 1 respectively at 25^0C. The value of s†an dard formation enthalpies
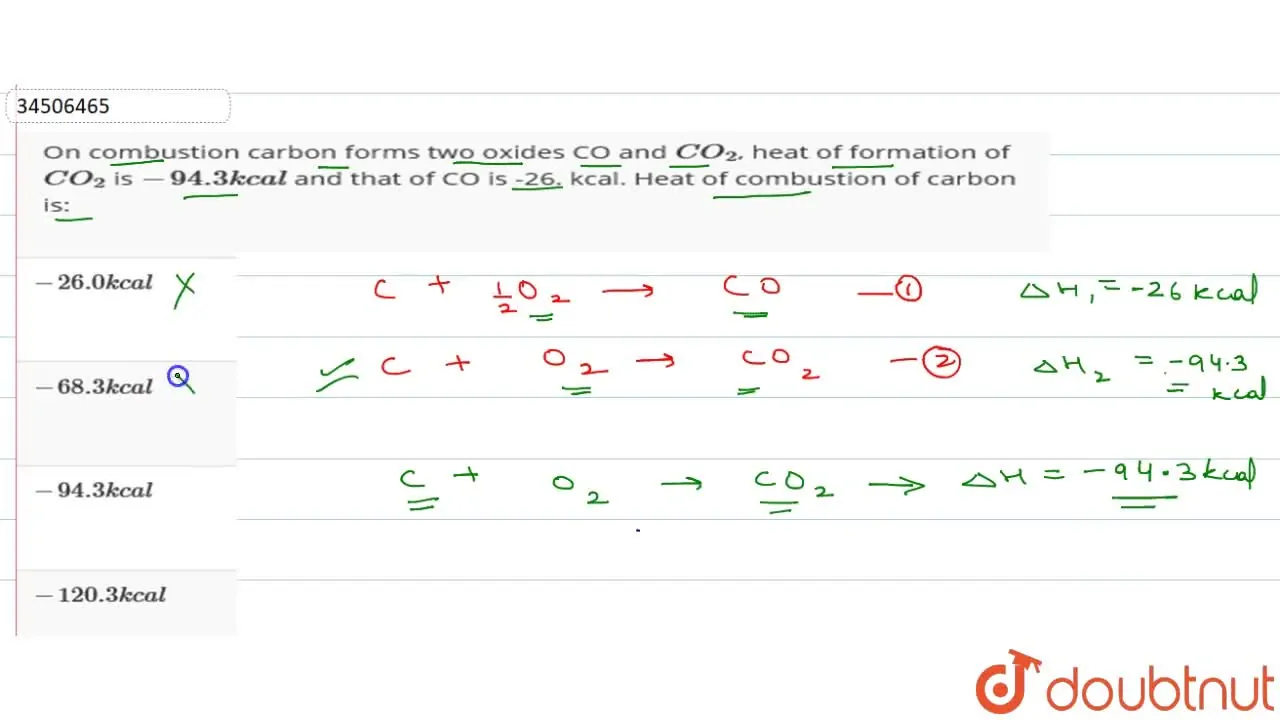
37. the heat of combustion of C,S carbondisulphide 393.3, 293.7 1108.78KJ.What will be heat of formation of carbondisulphide
The heat of combustion of carbon to CO2 is 393.5Kj/mol. The heat rrleased upon formtion of 35.2 g of CO2 from carbn and oxygem gad is
The heat of combustion of carbon to CO2 is 395.5kJ/mol. The heat released upon formation of 35.2 g of CO2 from carbon and oxygen gas is

Enthalpy of combustion of carbon to `CO_(2)` is `-393.5 kJ mol^(-1)`. Calculate the heat released upon formation of `35.2 g` of `CO_(2)` from carbon and dioxygen gas.
![Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of comb… | Heat energy, Chemical reactions, Exothermic reaction Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of comb… | Heat energy, Chemical reactions, Exothermic reaction](https://i.pinimg.com/474x/5c/b7/ee/5cb7ee448aac99cfa19825133b6bdbf6.jpg)
Total: 2 Average: 5/5] What is the heat of combustion? What is the definition of enthalpy of comb… | Heat energy, Chemical reactions, Exothermic reaction
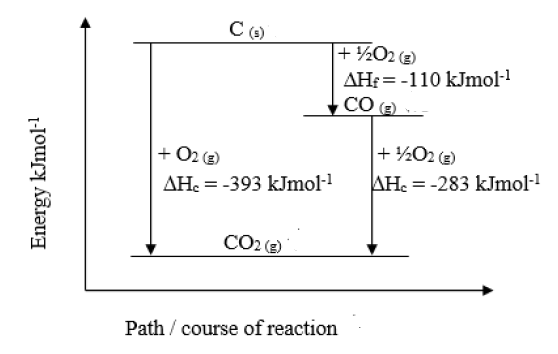
The heats of combustion of carbon and carbon monoxide are –393.5 and –283.5 kJ mol^–1, respectively. - Sarthaks eConnect | Largest Online Education Community